Cell Pouch System - Dramatic Diabetes Breakthrough in 2014?
Companies / Healthcare Sector Dec 14, 2013 - 04:27 PM GMTBy: Richard_Mills
 In a person with diabetes, the insulin producing beta cells in the pancreas are attacked by the immune system and destroyed, this leads to a deficiency of insulin and high blood sugar (glucose).
In a person with diabetes, the insulin producing beta cells in the pancreas are attacked by the immune system and destroyed, this leads to a deficiency of insulin and high blood sugar (glucose).
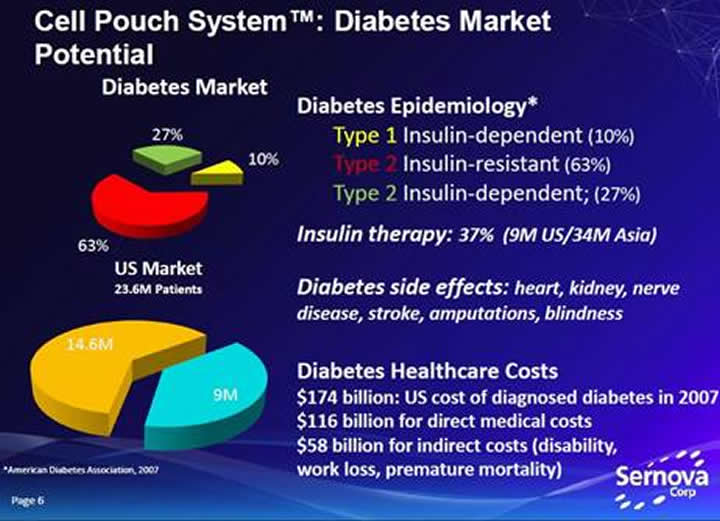
People with insulin-dependent diabetes (all of type-1 and about 27 percent of type-2 diabetics) lack the necessary amount of insulin in their bloodstreams. This can, and often does lead to irreversible damage to their hearts, blood vessels, eyes, kidneys, skin, feet and hearing.
Diabetes is not considered a high mortality condition, but it is a major risk factor for other causes of death and has an extremely high attributable burden of disability, for example; two percent of people with diabetes become blind and roughly ten percent develop severe visual impairment, fifty percent of people with diabetes die of cardiovascular disease.
There is no cure for diabetes. The Standard of Care for patients with reduced or missing critical hormones or proteins, such as insulin, is often monitoring and injecting these proteins multiple times a day.
Edmonton Protocol
Paul Lacey was a researcher at Washington University when, in 1972, he cured/restored normoglycaemia in some diabetic rats by transplanting islet cells (clusters of thousands of endocrine cells that include beta cells that produce insulin) from healthy rats into the livers of some diabetic rodents.
Over the next two decades researchers made hundreds and hundreds of attempts to apply the procedure to humans. Unfortunately no one was successful. By the early 1990’s most scientists had come to the conclusion that islet-cell transplantation was a lost cause.
Dr. James Shapiro, Dr. Jonathan Lakey and colleagues from the University of Alberta in Edmonton developed the Edmonton Protocol in the late 1990s.
The Edmonton Protocol is a method of implantation of pancreatic islets into the portal vein (a large vein returning blood from the abdomen to the liver), of the recipient's liver.
The first patient was treated in March of 1999. The protocol was published in the New England Journal of Medicine in July 2000. Considerable excitement was created by the report - the first seven patients were still insulin-independent after an average of 12 months.
How it works
Researchers use specialized enzymes to remove islets from the pancreases of recently deceased adults. The islets are then purified and counted.
Each transplant patient receives islets from one to three donors. X rays and ultrasound guide the placement of a thin, flexible tube called a catheter through a small incision in the upper abdomen and into the portal vein. The islets are then infused - pushed - slowly into the liver through the catheter.
The islets are kept from being destroyed by the recipient's immune system through the use of two immunosuppressant drugs as well as an antibody drug specifically used in transplant patients.
Once implanted, the beta cells in the transplanted islets begin to make and release insulin. However full islet function and new blood vessel growth from the new islets takes time so transplant recipients usually need to take insulin injections until the islets are fully functional.
Since 2000 several hundred people have received islet transplants – but by five years after the procedure, fewer than 10 percent of all patients are free of daily insulin supplementation.
Obstacles to Edmonton Protocol
The shortage of islets is a significant obstacle, organ donor rates are generally very low and especially so in Canada. Unfortunately, many of the pancreases that are donated are not suitable for extracting islets because they do not meet the selection criteria and islets are often damaged or destroyed during processing. The number of islet transplants that can be performed each year is therefore limited by a severe shortage of suitable islets.
Immunosuppressive medications have significant side effects and long-term effects are still not fully known. Immediate side effects may include mouth sores and gastrointestinal problems - an upset stomach and diarrhea.
Patients may also suffer from:
- Increased blood cholesterol, or blood fat, levels
- High blood pressure
- Anemia
- Fatigue
- Decreased white blood cell counts
- Decreased kidney function
- Increased susceptibility to bacterial and viral infections
- Increased risk of developing certain tumors and cancers
Unfortunately the autoimmune response that destroyed the transplant recipients' own beta cells in the first place can happen again.
There are issues with infusion of islets into the portal vein, an immediate blood mediated inflammatory reaction (IBMIR) causes over 50 percent of islets to die within hours or days following infusion into the blood.
Another issue is that the liver site is associated with islet transplant related procedural complications including catheter-induced hemorrhage and thrombosis. Additional inhibiting factors include difficulty in imaging the islets, an inability to remove the transplanted islets and the limited number of islet transplants a patient can receive.
The State of Islet Transplantation today
From the report ‘Improvement in Outcomes of Clinical Islet Transplantation: 1999–2010’ the author found on diabetesjournals.org comes the following…
“Allogeneic islet transplantation offers a minimally invasive option for β-cell replacement in people with type 1 diabetes complicated by recurrent severe hypoglycemia and/or marked glycemic lability.
Before 1999, less than 10% of islet transplant recipients achieved insulin independence. In 2000, the Edmonton Protocol for islet transplantation achieved insulin independence in seven consecutive participants who received islets from more than one donor under a steroid-free immunosuppression regimen. After this proof-of-concept success, islet transplant programs expanded in North America and elsewhere. These centers have offered evolving strategies of islet preparation and immunosuppression, although the limited resources available have prevented anything but independent Phase I/II attempts to standardize processes, achieve success, and stabilize outcomes.
Even in the absence of insulin independence, an islet transplant can protect type 1 diabetic recipients from severe hypoglycemic episodes as long as residual islet graft function is maintained, as proven by restoration of C-peptide production. Despite this compelling rationale, islet transplantation for type 1 diabetes has produced variable success and elusive durability, has frequently required multiple donor organs, and has balanced one disease load - severe hypoglycemia - with another - long-term immunosuppression.”
A search has been on for an alternative site for islet transplantation as well as for an optimal medical device in which to implant the islets. Several subcutaneous devices have previously been developed for islet transplantation but from a preclinical and clinical perspective the results from these products have been generally disappointing.
Current cell therapy is limited to poor cell survival, inappropriate delivery of hormones, a lack of available donors and cells.
Sernova Corp. TSX.V – SVA
Sernova Corp is a Phase I/II clinical stage company developing medical technologies for the treatment of chronic debilitating metabolic diseases to replace proteins or hormones in short supply within the body.
Sernova is currently developing two novel and closely integrated, proprietary platform technologies.
The first proprietary platform technology is the Cell Pouch System™.
Sernova’s Cell Pouch System™ is a versatile and scalable, first-in-class implantable medical device made entirely of FDA approved materials. The Cell Pouch System™ provides a natural “organ-like” environment rich in tissue matrix and micro-vessels. This is the ideal environment for therapeutic cells to thrive which then release proteins and/or hormones as required. The Cell Pouch System™, being thin and typically smaller than a business card, fits easily under the skin with virtually no visibility.
Due to the enormous market and potential for significantly improved patient treatment Sernova’s first product focus is on diabetes - islet cell transplantation is the only known therapy that can reduce or eliminate the side effects associated with this chronic disease.
At this time there is no approved device to house and protect therapeutic cells transplanted into a patient. The Standard of Care for patients with insulin-dependent diabetes is monitoring and injecting insulin multiple times a day.
Think of the Cell Pouch System™ as a potential natural insulin producing pump with the added benefit of fine-tuned glucose control with no need to replenish the insulin. When placed under the skin and filled with islets it develops pancreas-like characteristics taking over normal glucose control. A key feature of the device is its ability to develop tissue matrix and natural micro-vessels, thought to be essential for the long-term survival and function of therapeutic cells.
The second proprietary platform technology is Sertolin™ - a cell-based technology providing an immune-privileged environment for donor cells which reduces or eliminates the need for anti-rejection drugs.
Sertolin, when combined with therapeutic cells, protects them from attack by the immune system. The combination of these protector and therapeutic cells leads to long-term functional survival of the therapeutic cells without drug therapy. The Sertolin™ technology has the potential to reduce or eliminate the need for expensive lifelong daily anti-rejection drug cocktails that make subjects susceptible to serious infections.
Cell Pouch System™ Phase I/II Human clinical study to treat Type-1 diabetes
In September 2010, following an extensive review of Sernova's preclinical data, Dr. Shapiro MD, Ph.D. FRCS (Eng) FRCSC joined Sernova Corp's Scientific Advisory Board.
On August 16th 2012 Sernova, and the University of Alberta, announced the treatment of the first patient with insulin-producing islets transplanted into Sernova's Cell Pouch System™ in a Phase I/II clinical study to treat Type-1 diabetes.
The clinical study is being led by Dr. James Shapiro, Professor of Surgery and Medicine, University of Alberta and Director, Clinical Islet Transplant Program.
Sernova announced, on September 26th 2013, results from the ongoing Cell Pouch System™ Phase I/II clinical study.
"We are really encouraged by the initial results of this ground-breaking study. The preliminary findings that human islets survive under the skin within the Cell Pouch pave the way for our ongoing studies in patients that will now test how effective this new approach will be. Importantly, the islets were shown to be residing within a natural tissue matrix in the device, and were nicely integrated with micro-vessels, and stained for insulin, glucagon, somatostatin and polypeptide at the 30 day time point." Dr. James Shapiro, principal investigator Sernova Cell Pouch System™ Phase I/II clinical study

Efficacy results are expected from the Cell Pouch System™ Phase I/II clinical study early in 2014.
An enabling platform
Sernova is exploring the additional utility of the Cell Pouch System™ as an enabling platform for a range of therapeutic cell types. The technology could be used for a patient’s own cells (autograft), or a donor’s cells (allograft).
The therapeutic Cells placed into the device may also be cells that can be a source to treat millions of patients such as stem cells (stem cells have the ability to differentiate into other cell/tissue types) or xenogeneic (derived or obtained from an organism of a different species) cells. Sernova is talking to stem cell companies and suppliers of porcine (pig insulin is almost identical to human insulin with the exception of one amino acid) cells to ultimately expand the supply of islet cells that the company can use to develop its therapy.
Sernova’s products are also designed to allow for multiple market expansion opportunities within each therapeutic area. For example, the technology would be beneficial if it provided a simple reduction in the number of daily therapeutic injections a patient must take; however, there is the possibility that it could even essentially ‘cure’ the disease through natural release and regulation of the therapeutic proteins or hormones.
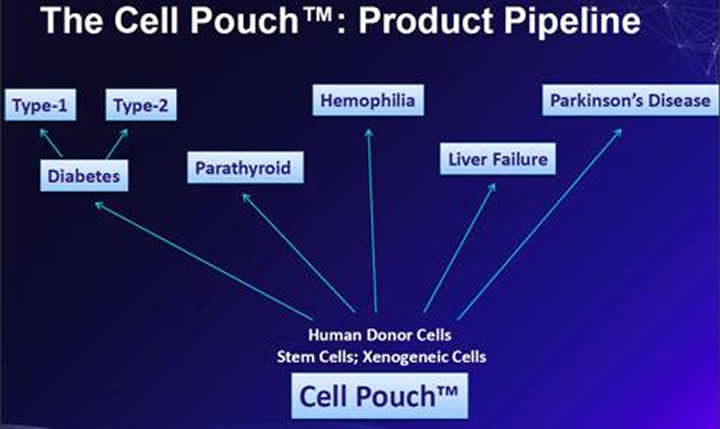
The Cell Pouch™ could be used for any chronic disease where a deficient or missing protein or hormone can be replaced by therapeutic cell transplantation.
Sernova has recently announced a collaboration with Medicyte GmbH to develop a product for Haemophilia.
“Sernova is unique in having the only proprietary human scalable technology solution for therapeutic cells which can thrive within its Cell Pouch™ in a natural tissue matrix rich in blood vessels and protected by Sertolin™ from immune system attack.” Dr. Philip M. Toleikis BA, MSc, PhD President and CEO Sernova Corp.
Sernova’s current targeted markets
Cell therapies can cost $10,000 each and the Cell Pouch™ is thought to have a cost of $10,000, more study is needed as trials progress but SVA’s total product combination could bring in as much as $30,000 to $50,000 a patient.
Diabetes
Worldwide expenditures on insulin are estimated to be over U.S. $15 billion annually. Total costs of diagnosed diabetes in the United States was $245 billion.
About 347 million people worldwide have diabetes. The World Health Organization (WHO) projects that diabetes will be the 7th leading cause of death in 2030.
Haemophilia
Patients with hemophilia A have a defective factor VIII gene. Currently, the number of people with hemophilia in the United States is estimated to be about 20,000, an estimated 400,000 people worldwide are living with hemophilia and only 25% receive adequate treatment.
Patients receive prophy-laxis factor replacement therapy two to three times a week. Prophylactic therapy (prevention therapy) involves three infusions of Factor VIII each week at the hospital at a cost of about $260,000 each year.
The broader hemophilia market was $8.5 billion in 2011 and is expected to grow to $11.4 billion in 2016.
Conclusion
The holy grail of diabetes and hemophilia A treatment is for patients not to have to take injections or infusions.
Next year, 2014, could be a break-through year for SVA, its technologies and for diabetes and hemophilia sufferers.
"The findings in these initial patients parallel the results in Sernova's multiple small and large animal safety and efficacy studies of the Cell Pouch. These showed an exemplary safety profile, and that islets were well- vascularised with microvessels and produced insulin. In addition, the islet-rich Cell Pouches then went on to show long-term glucose control in the diabetic animals." Dr. David White, Professor Emeritus and Chair of Sernova's Scientific Advisory Board talking about Sernova’s September 26th 2013, results from the ongoing Cell Pouch System™ Phase I/II clinical study
Here’s how I think things are going to play out for Sernova over 2014 and beyond:
- Board appointments
- Cell Pouch System™ Phase I/II clinical study efficacy results
- In-house licensing deals with major universities
- Regional licensing deal
There is no market cycle for drug, bio-technology and bio-med device stocks. The need is always there and demand is growing at an alarming rate while at the same time big pharma’s number of patents and pipeline of new devices and drugs has fallen off a cliff. This “patent cliff” has shifted the playing field so much so that any new and exciting drug or device will be snapped up at a much earlier stage than previously thought possible.
Sernova Corp. (TSX.V-SVA) and it’s Cell Pouch System™ human trial PhaseI/II clinical study efficacy results should be on everyone’s radar screen. It’s definitely on mine.
Is Sernova on your radar? If not, it should be.
By Richard (Rick) Mills
If you're interested in learning more about the junior resource and bio-med sectors please come and visit us at www.aheadoftheherd.com
Site membership is free. No credit card or personal information is asked for.
Richard is host of Aheadoftheherd.com and invests in the junior resource sector.
His articles have been published on over 400 websites, including: Wall Street Journal, Market Oracle, USAToday, National Post, Stockhouse, Lewrockwell, Pinnacledigest, Uranium Miner, Beforeitsnews, SeekingAlpha, MontrealGazette, Casey Research, 24hgold, Vancouver Sun, CBSnews, SilverBearCafe, Infomine, Huffington Post, Mineweb, 321Gold, Kitco, Gold-Eagle, The Gold/Energy Reports, Calgary Herald, Resource Investor, Mining.com, Forbes, FNArena, Uraniumseek, Financial Sense, Goldseek, Dallasnews, Vantagewire, Resourceclips and the Association of Mining Analysts.
Copyright © 2013 Richard (Rick) Mills - All Rights Reserved
Legal Notice / Disclaimer: This document is not and should not be construed as an offer to sell or the solicitation of an offer to purchase or subscribe for any investment. Richard Mills has based this document on information obtained from sources he believes to be reliable but which has not been independently verified; Richard Mills makes no guarantee, representation or warranty and accepts no responsibility or liability as to its accuracy or completeness. Expressions of opinion are those of Richard Mills only and are subject to change without notice. Richard Mills assumes no warranty, liability or guarantee for the current relevance, correctness or completeness of any information provided within this Report and will not be held liable for the consequence of reliance upon any opinion or statement contained herein or any omission. Furthermore, I, Richard Mills, assume no liability for any direct or indirect loss or damage or, in particular, for lost profit, which you may incur as a result of the use and existence of the information provided within this Report.
Richard (Rick) Mills Archive |
© 2005-2022 http://www.MarketOracle.co.uk - The Market Oracle is a FREE Daily Financial Markets Analysis & Forecasting online publication.



